Biotechnological Leading Company
Bio Products
CTM
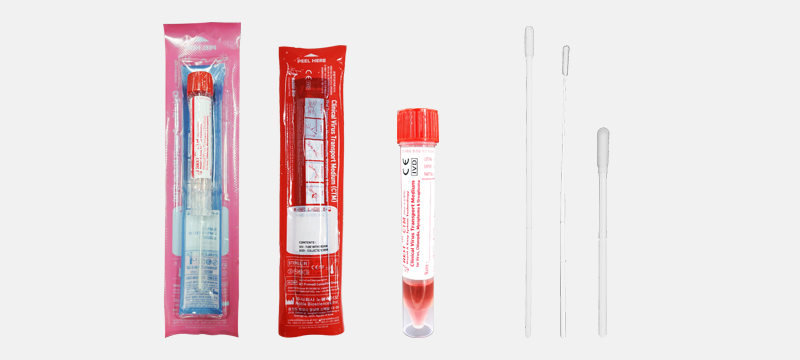
Clinical Transport Medium (CTM) manufactured by a partner company, Noble Bio Co., Ltd
Clinical Transport Medium(CTM) is intended for the collection and transport of clinical
specimens containing Virus, Chlamydia, Mycoplasma, Ureaplasma from the collection site.
The test procedures for quality control are based upon the quality control methods described in CLSI M40-42 and others.
Specimens in CTM transport medium are stable for 48 hours at room temperature.
This CTM collection and transport medium is certified as Biopsy Procedure Kit,
class ll with product-license number 15-211 by Ministry of Food and Drug Safety.
(Manufacturing authorization number : JAEIN 15-211)